Case Study: Electrochemical Lipid Membrane Kinetics
Introduction
This case study shows the development of a high through-put microfluidic platform utilising an artificial lipid membrane constructed such to mimick a bacterial cell wall. The platform uses the electrochemical method of cyclic voltmmetry to measure and characterise the membrane. The cyclic voltammograph produces a fingerprint of the membrane. We systemmatically presented a library of compounds to be screened and evaluated to the membrane so that their kinetic interaction can be observed via the voltammogram.
Key Points
Completed System

Technologies
Customer

External Links & Publications
Artificial Lipid Membrane
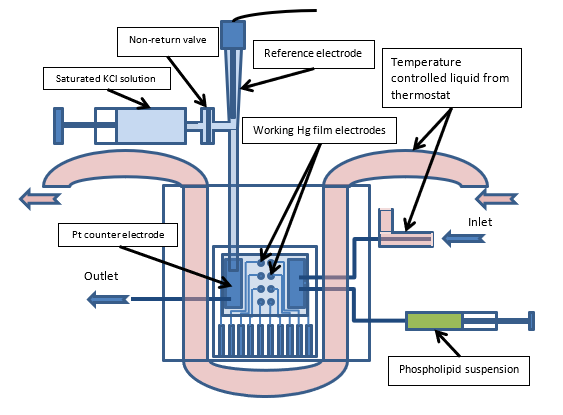
A micro-electrode (or an array of micro-electrodes) created using lithographic techniques were positioned in a temperature controlled microfluidic flow cell. The electrode arrays comprise working, counter and reference. Using a variety of phosphorlipid membrane suspensions and under the correct flow and electrcohemical conditions a single layer and double layer phospholidpid membrane was formed onto the eletrode. Using different solutions and different electrochemical conditions membranes could be cleaned or removed allowing a fully automated system.
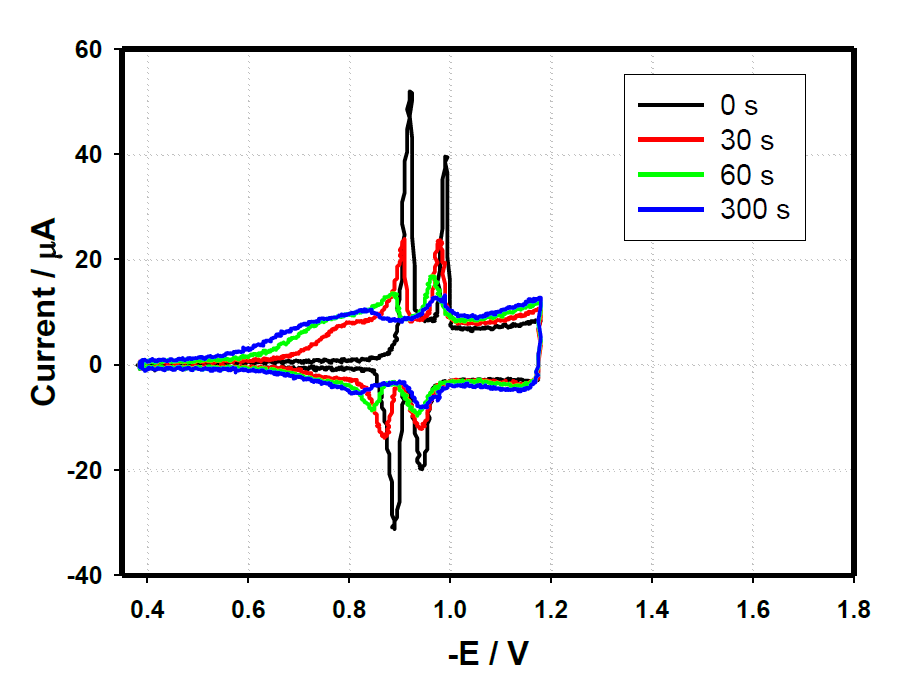
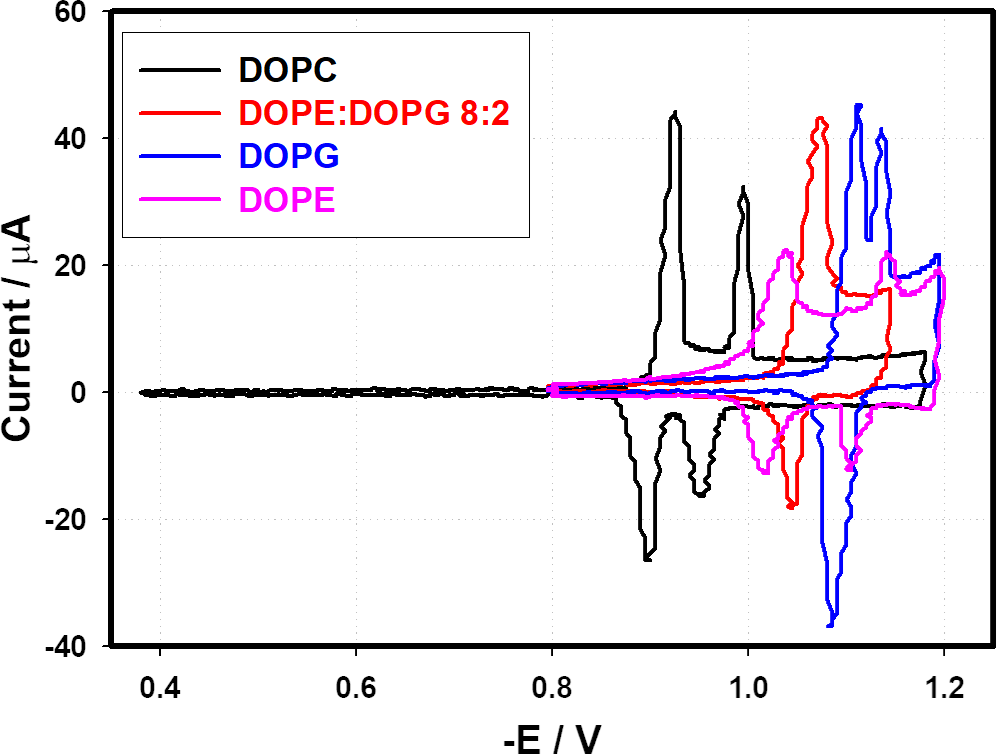
Membrane Kinetics
Many antimicrobial inhibitors function by permeabilising the cytoplasmic membrane. Bacterial membranes have high contents of negatively charged lipids like phosphatidylglycerol (PG) or diphosphatidylglycerol (DPG or cardiolipin), which are the most abundant anionic phospholipids of cytoplasmic bacterial membranes. These phospholipids are particularly prominent in a number of gram-positive bacteria.
Using electrochemical measurements a characteristic "fingerprint" of membrane could be observed. When compounds were presented we could monitor the kinetic interaction of the compound with the membrane and therefore characterize the compounds effectiveness and its action.
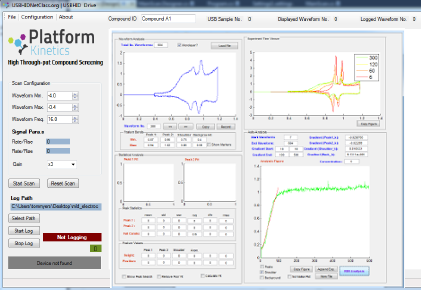
Software
Custom desktop software was developed which allowed the system to be automated for producing the electrodes, loading the compounds and performing the electrochemical measurements. Once a compound had been screened then data extraction and analysis was automatically performed on the collected data and each compound ranked and the action of the compound reported. The user could then choose to perform concentration studies on the most promising compounds.